

You’ve probably seen those little FDA labels on medical devices, from simple thermometers to high-tech implants. But have you ever wondered what those labels really mean? Is an FDA-approved device better than a cleared one? And does it even matter?
If you’re like most people, you probably don’t give it much thought. But understanding the difference between FDA approved vs cleared can help you make more informed decisions about the medical devices you use – and ultimately, your health.
Let’s break it down…
Before we get into the nitty-gritty, let’s familiarize ourselves with the FDA. The U.S. Food and Drug Administration is a federal agency responsible for safeguarding public health. They’re like the watchful guardians of the products we use and consume daily—everything from medications and medical devices to food and cosmetics.
The FDA is in charge of ensuring that medical devices are safe and effective for their intended use. They do this through a rigorous review process that evaluates the scientific evidence provided by manufacturers.
The FDA’s involvement doesn’t stop at approval or clearance. They continue to monitor devices after they’re on the market, watching for any safety concerns or adverse events. Think of them as your health watchdog, always on the lookout for potential risks.
Not all medical devices undergo the same level of scrutiny. The FDA classifies medical devices into three categories based on their risk level:
It’s important to note that this classification isn’t set in stone. The FDA can change a device’s classification if new risks emerge or if its intended use changes.
FDA clearance doesn’t mean the FDA has tested the device themselves. Instead, it means the manufacturer has demonstrated that their device is “substantially equivalent” to a device that’s already on the market and considered safe and effective. This process is called premarket notification, also known as the 510(k) pathway.
The FDA reviews the manufacturer’s data, comparing the new device to a “predicate” device. If the FDA determines that the new device is as safe and effective as the predicate, they “clear” it for marketing.
For some low- to moderate-risk devices that don’t have a suitable predicate, the FDA offers a pathway called De Novo classification. This allows the FDA to classify a novel device and then clear it for marketing through the 510(k) process.
FDA approval is the gold standard for medical devices. It means the FDA has conducted a thorough review of the manufacturer’s clinical data and determined that the device is safe and effective for its intended use. This process, called premarket approval (PMA), is much more rigorous than the 510(k) process.
Manufacturers must submit extensive evidence from clinical trials, demonstrating the device’s safety and effectiveness in human subjects. The FDA also inspects the manufacturing facilities to ensure they meet quality standards.
Feature | FDA Cleared | FDA Granted | FDA Approved |
Risk Level | Class II | Class I or II | Usually Class III |
Review Process | Premarket Notification (510k) | De Novo Classification | Premarket Approval (PMA) |
Evidence Required | Substantial Equivalence to Predicate | Safety & Effectiveness for Novel Device | Safety & Effectiveness with Clinical Data |
Review Time | Typically Shorter | Varies | Typically Longer |
The 510(k) submission process is how manufacturers obtain FDA clearance for their medical devices. Here’s a simplified overview of the steps involved:
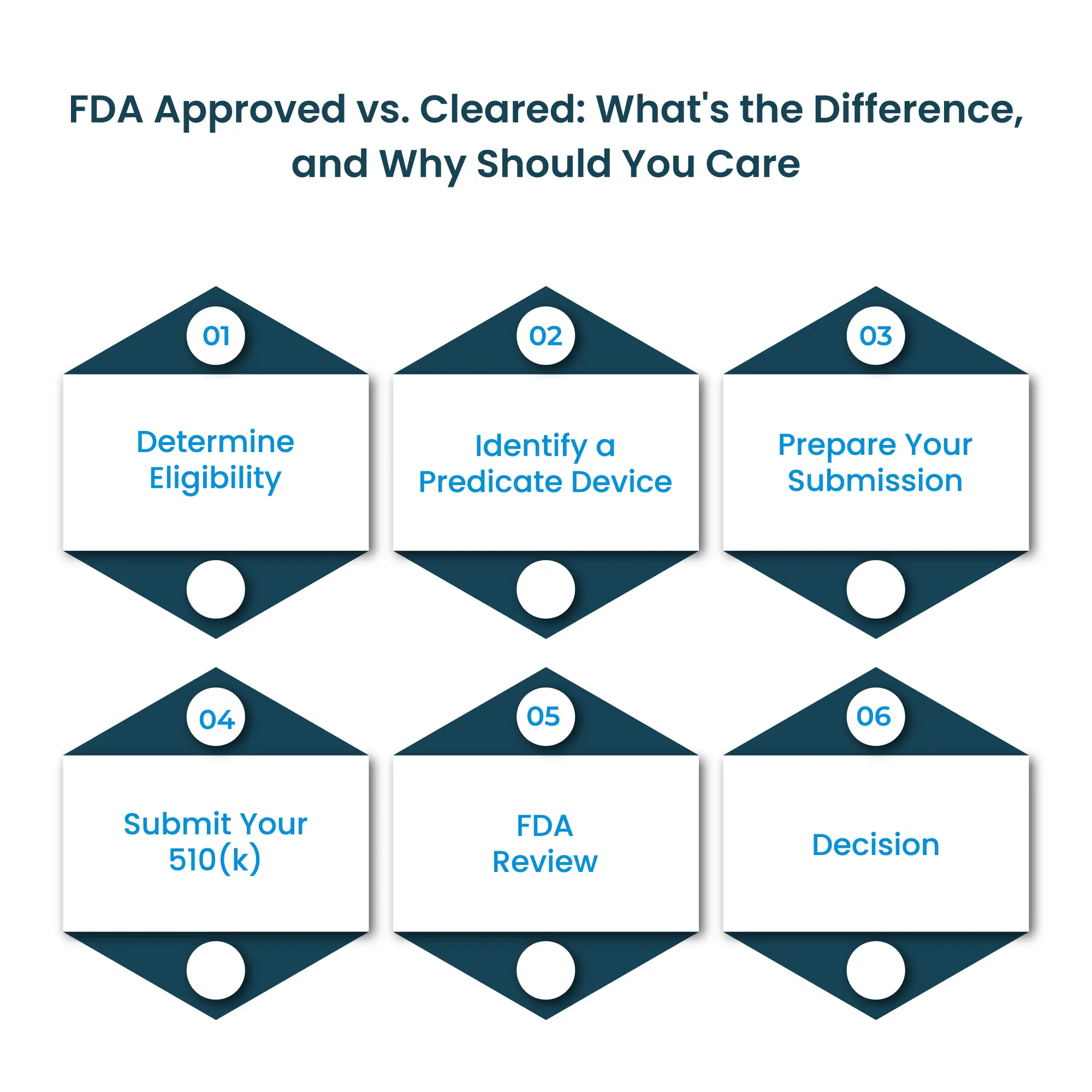
The cost of a 510(k) submission can vary widely depending on the complexity of your device, the amount of testing required, and whether you use consultants or in-house staff. Here’s a general range to give you an idea:
The PMA application is a comprehensive document submitted to the FDA to demonstrate the safety and effectiveness of a high-risk medical device. It’s a complex process that typically takes about six months or more to complete. Here’s what you need to know:
The PMA application must include a wide range of information, including:
The FDA review process for PMA applications typically takes at least 180 days. However, it can take significantly longer, depending on the complexity of the device and the quality of the submitted data. The FDA may also request additional information or clarification from the manufacturer, which can further extend the timeline.
Obtaining FDA approval through the PMA pathway is a major milestone for medical device manufacturers. It demonstrates that the device has met the highest standards for safety and effectiveness, giving healthcare providers and patients confidence in its use.
While both terms relate to the FDA’s oversight of medical devices, they represent very different levels of regulatory scrutiny:
FDA Registered
FDA Approved
In the end, knowing whether a medical device is FDA-cleared or approved can empower you to make smarter decisions about your healthcare. While both types of devices must meet safety standards, FDA approval signifies a more rigorous evaluation process, particularly crucial for high-risk devices. So, the next time you see those FDA labels, take a moment to decipher their meaning. It could make all the difference in your health journey.
Talk to an Expert Now